An Active and Regenerable Nanometric High-Entropy Catalyst for Efficient Propane Dehydrogenation
Abstract
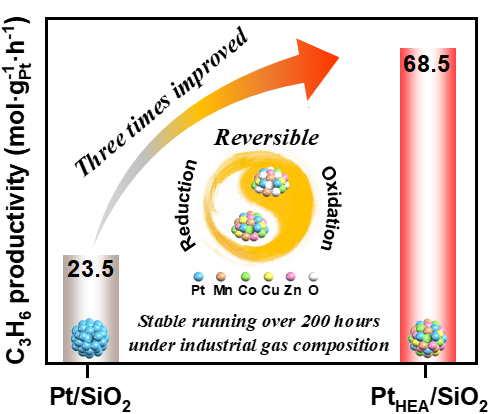
Propane dehydrogenation (PDH) is crucial for propylene production, but commercially employed Pt-based catalysts face susceptibility to deactivation due to the Pt sintering during reaction and regeneration steps. Here, we report a SiO2 supported nanometric (MnCoCuZnPt) high-entropy PDH catalyst with high activity and stability. The catalyst exhibited a super high propane conversion of 56.6% with 94% selectivity of propylene at 600 °C. The propylene productivity reached 68.5 molC3H6·gPt-1·h-1, nearly three times that of Pt/SiO2 (23.5 molC3H6·gPt-1·h-1) under a weight hourly space velocity of 60 h-1. In a high-entropy nanoparticle, Pt atoms were atomically dispersed through coordination with other metals and exhibited a positive charge, thereby showcasing remarkable catalytic activity. The high-entropy effect contributes to the catalyst a superior stability with a low deactivation constant of 0.0004 h-1 during 200 hours of reaction under the industrial gas composition at 550 °C. Such high-entropy PDH catalyst is easy regenerated through simple air combustion of deposited coke. After the fourth consecutive regeneration cycle, satisfactory catalytic stability was observed, and the element distribution of spent catalysts almost returned to their initial state, with no detectable Pt sintering. This work provides new insights into designing active, stable, and regenerable novel PDH catalysts.

