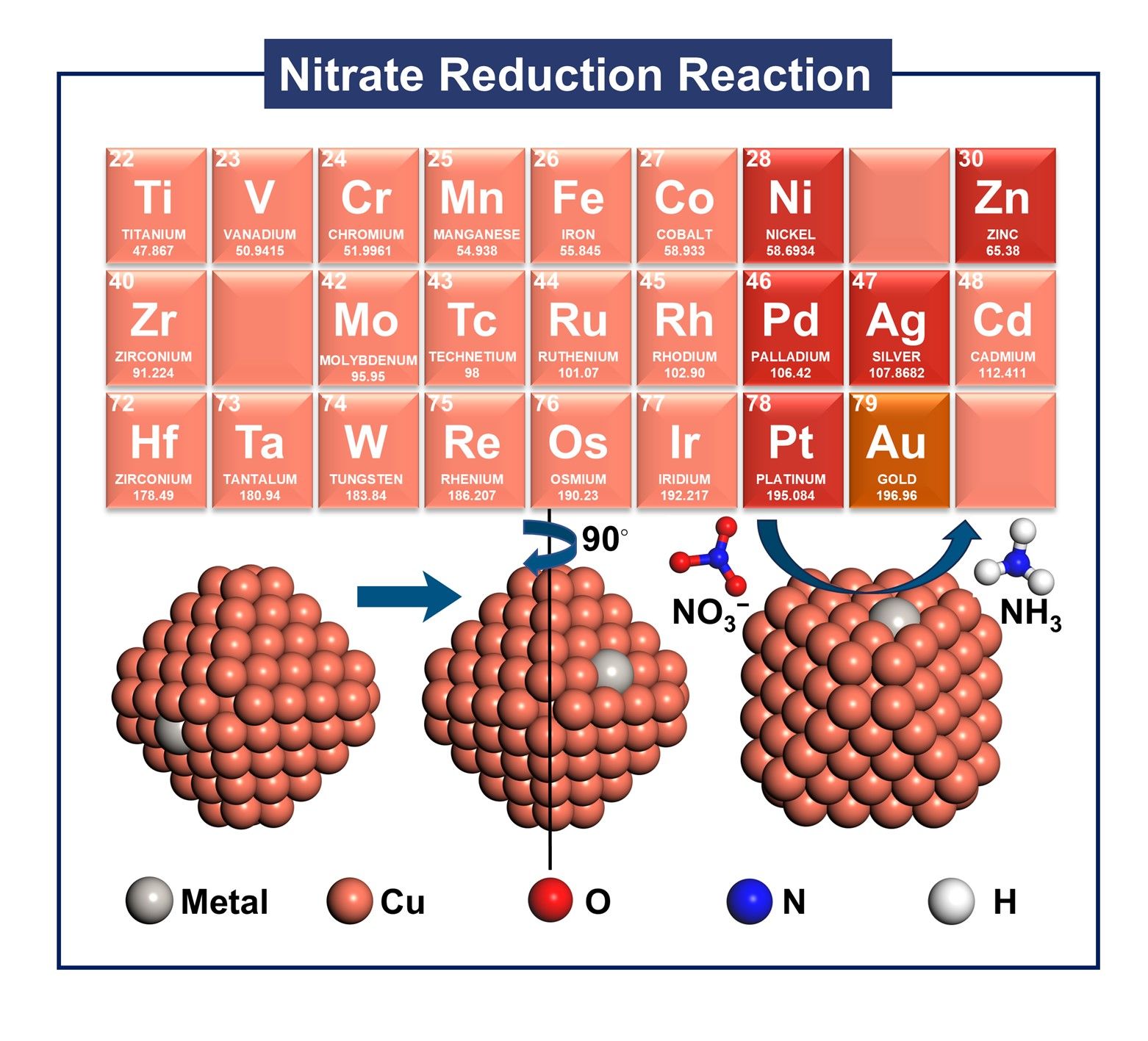
Ab initio study on doped Cu138X2 nanoparticles with dispersed surface-active sites for enhanced electrocatalytic nitrate reduction to ammonia
Abstract
Copper nanoparticles (NPs) exhibit significant potential in electrocatalysis owing to their tunable electronic structure and abundant surface-active sites. However, the multifaceted complexity of Cu-based nanoparticles induced by heteroatom dopants fundamentally limits our ability to decipher the nitrate-to-ammonia electroreduction (NO3RR) mechanism, creating a critical knowledge gap that obstructs the targeted engineering of advanced catalysts with atomic precision. In this study, we employed first-principles calculations to design a series of highly surface-dispersed transition metal-doped Cu138X2 (X = Ag, Au, Pd, Zn, Ni, Pt) bimetallic electrocatalysts for nitrate reduction to ammonia, based on the structure of Cu140 NPs. Theoretical analysis revealed that Cu138Au2 exhibits significant advantages in NO3RR, with a remarkably low limiting potential of -0.20 eV. Additionally, significant energy barriers were observed for the formation of by-products NO and NO2 on Cu138Au2, ensuring high selectivity towards ammonia. For Cu-based catalysts, we propose that the *NO2→*HNO2 step is critical and can serve as a descriptor for rapid screening of Cu-based catalysts. This study not only provides important insights into the research of Cu NPs as NO3RR electrocatalysts but also establishes a theoretical foundation for enhancing their catalytic performance through surface modification or compositional tuning.

