Cathode-anode synergy electrosynthesis of propanamide via a bipolar C-N coupling reaction
Abstract
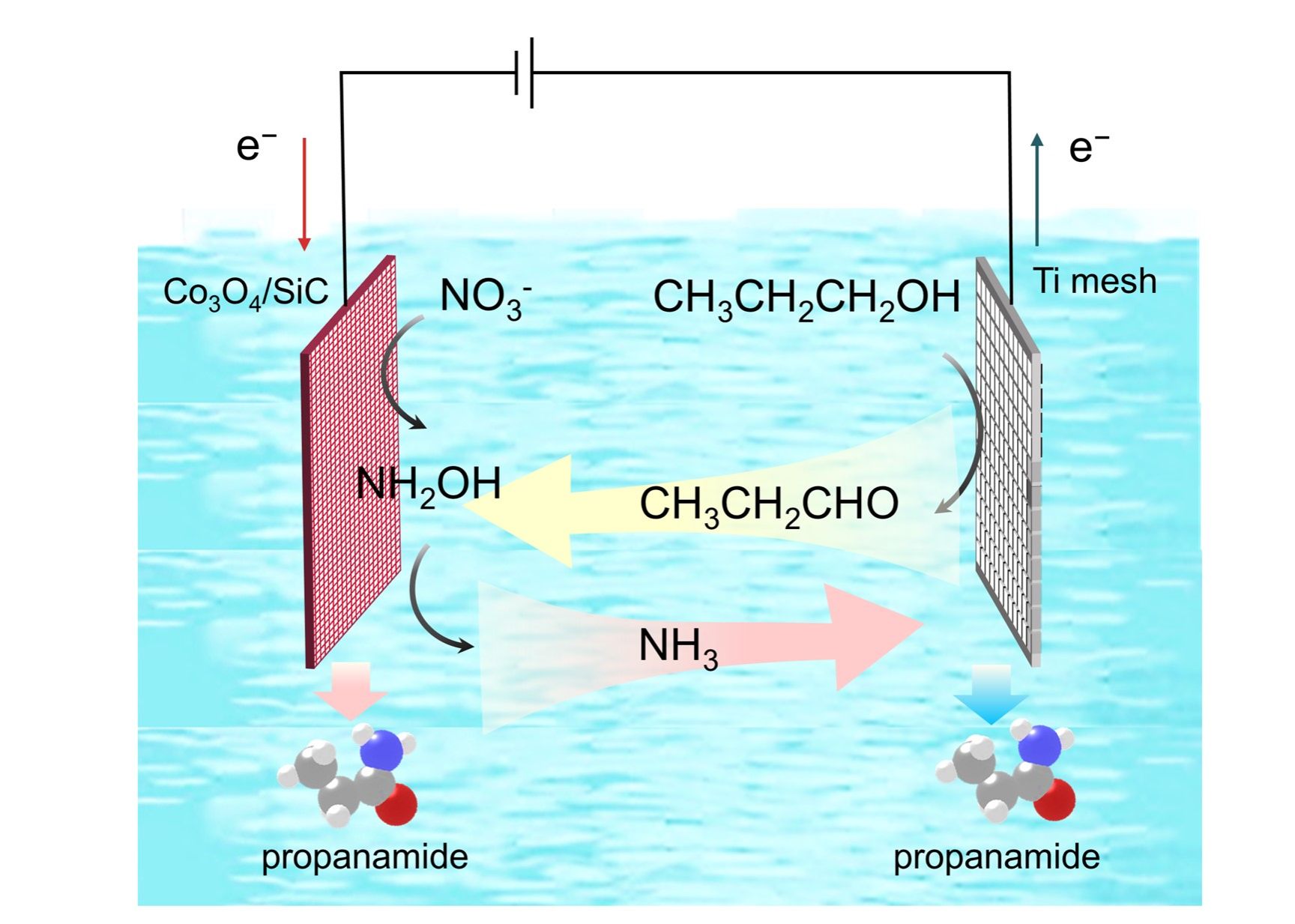
Propanamide is a crucial synthetic intermediate in pharmaceuticals for preparing antibacterial and anticancer drugs. Conventional synthesis of propanamide involves the reaction of carboxylic acid derivatives with amines, which requires harsh reaction conditions, leading to an unfavorable environmental footprint. Here, we present a cathode-anode synergistic electrochemical strategy to transform nitrate and n-propanol into propanamide under ambient conditions, where both the cathode catalyst Co₃O₄/SiC and the anode catalyst Ti contribute distinctively to the electrochemical process. The CH3CH2CHO produced at the Ti anode can diffuse and react with the adsorbed intermediate *NH2OH on the surface of the cathode catalyst to form propanamide. The synergistic reactions at both electrodes collectively enhance the efficiency of propanamide synthesis. This design enables efficient propanamide production in a flow cell at the gram scale, a remarkable yield of 986.13 μmol/(cm²·h) at current densities of up to 650 mA/cm². Our reports present a new option for environmentally friendly C-N bond synthesis, and the insights can be useful to the electrosynthesis of a wider scope of amides.

