Mitigating jahn-teller effect of Mn-based layered oxide cathodes for sodium ion battery by regulation of coordination chemistry
Abstract
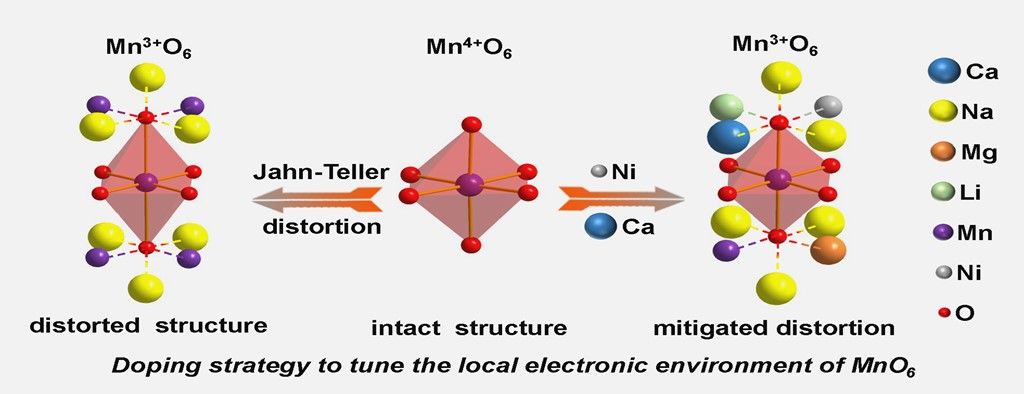
P2-type Mn-based layered oxide cathode materials are competitive candidates for sodium ion batteries (SIBs), which are expected to be widely used in large-scale electrochemical energy storage applications due to their easy availability. However, MnO6 octahedra centered around Mn³⁺ are inclined to adverse phase transitions and lattice oxygen loss under high operating voltages, which markedly compromise capacity and cycling stability. Here, a configurational entropy tuning strategy has been proposed to optimize the P2-type Na0.8Li0.17Mg0.18Mn0.66O2 (LMM) cathode. The as-synthesized cathode material Na0.8Li0.17Ca0.025Mg0.12Ni0.05Mn0.66O2 (LMCNM), conforms to the standard P63/mmc crystal phase. Impressively, this material exhibits a capacity retention rate of 92% after 100 cycles at a 0.4 C rate (where 1C = 125 mA h g-1) and demonstrates minimal volume change (0.94%) during charge-discharge cycles at higher working voltages (2.0-4.3V). In-situ X-Ray powder Diffraction (XRD), ex-situ X-ray photoelectron spectroscopy (XPS), and computational analyses collectively indicate that through the charging and discharging process of LMCNM, there is no obvious Jahn-Teller distortion, while there is clear evidence for charge compensation from Mn3+ to Mn4+. Furthermore, partial reversible anionic redox has been achieved through co-doping with Ca and Ni to harmonize expressive stability and high capacity.

