Steering amide synthesis from acetonitrile by electrochemically derived active superoxide
Abstract
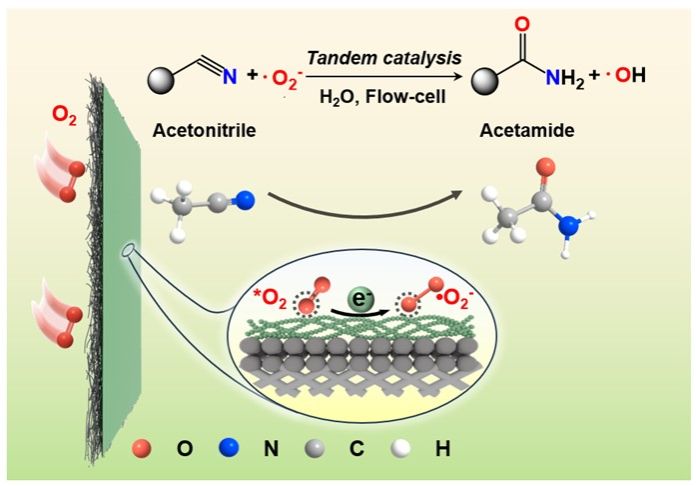
Amides with unique carbonyl-nitrogen linkage are fundamental functional groups that are active and essential for a wide range of natural and synthetic products, pharmaceuticals, agrochemicals, and key intermediates. Amide bond formation is one of the most fundamental reactions in chemistry. Conventional amide synthesis faces challenges of poor atom economy and reliance on hazardous reagents. Herein, we present a tandem electro-thermal catalytic pathway for the synthesis of acetamide from acetonitrile, leveraging electrochemically generated superoxide radicals to steer oxygen insertion under ambient conditions. The superoxide radicals were formed via controlled electroreduction of molecular oxygen. In 1 M K2CO3 electrolyte containing methanol, we achieved over 94% selectivity towards acetamide at a total current density up to 104 mA cm-2 , with a productivity of 878 μmol cm-2 h -1 . Mechanistic studies reveal that the superoxide radicals stabilized by a buffered alkaline environment facilitated the nucleophilic C-O coupling at the nitrile group, while the presence of methanol quenched the hydroxyl radicals and effectively suppressed the over-oxidation of acetamide to acetate. This coupled electro-thermal catalysis enables the synthesis of a large variety of amides, and may inspire more complex organic synthesis through active oxygen species-mediated process.

