Fe3+/Fe2+-Tuned Iiron oxides for enhanced interfacial nitrate activation toward efficient electrochemical ammonia production
Abstract
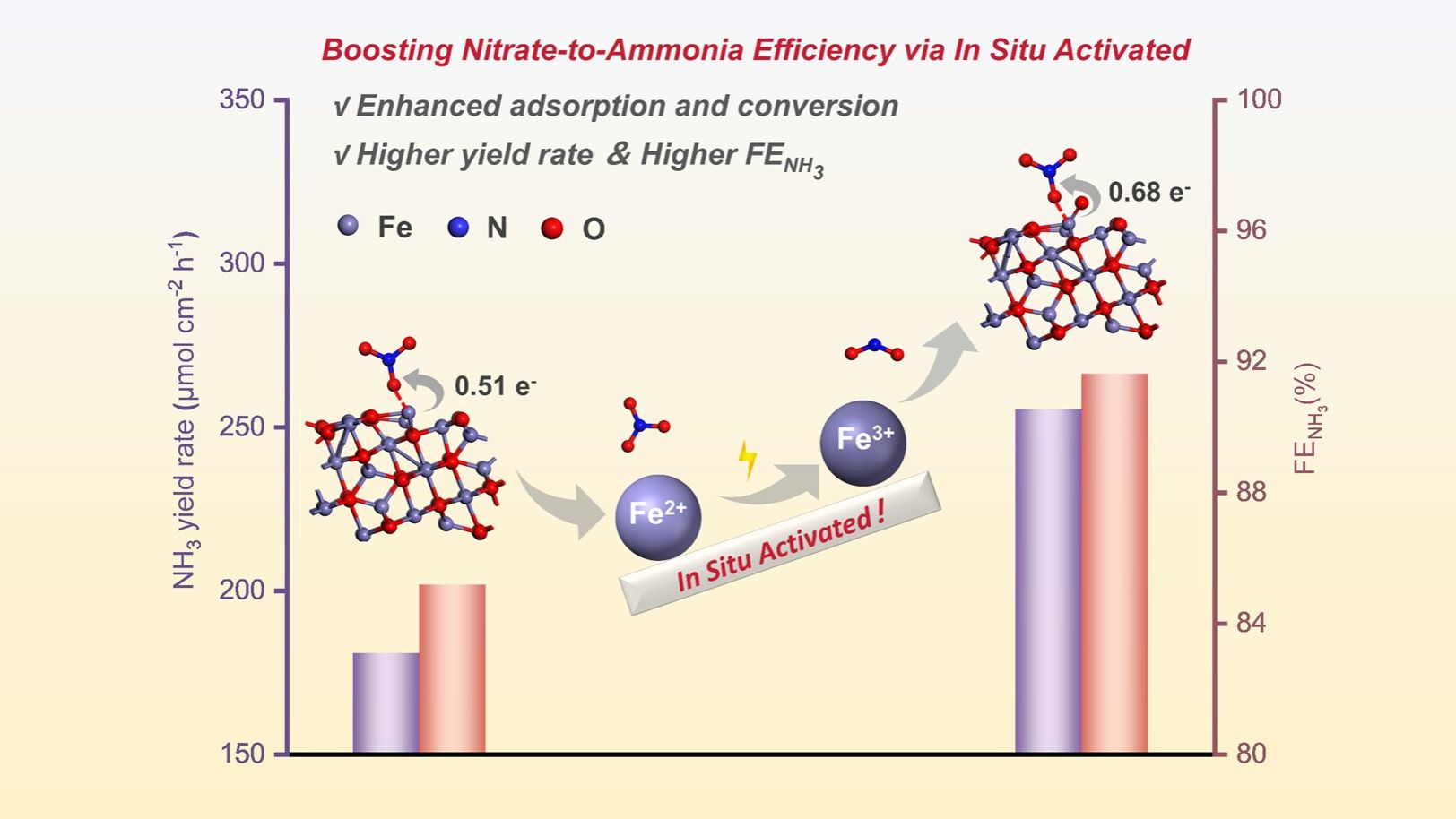
The electrochemical nitrate-to-ammonia conversion (NO3RR) offers a sustainable route for nitrogen utilization, yet its efficiency is limited by poor nitrate adsorption and sluggish reaction kinetics. Here, we present a mesoporous FeOx electrocatalyst (meso-FeOx) with dual geometric and electronic optimization for enhanced NO3RR performance. Through an in situ electrochemical pre-activation strategy (-1.3 V vs. RHE), the resulted FeOx catalyst, meso-FeOx-e1.3, achieves a great ammonia yield rate of 255.40 μmol cm-2 h-1 with a Faradaic efficiency for ammonia (FENH3) of 91.63% at -1.0 V vs. RHE, outperforming most previously reported Fe-based catalysts. X-ray Photoelectron Spectroscopy and theoretical calculations reveal that cathodically tuned Fe3+/Fe2+ redox pairs dynamically regulate the adsorption and activation of nitrate, while the well-defined mesoporous structure ensures efficient mass transport. This work demonstrates a synergistic design principle—combining nanostructural engineering with dynamic electronic modulation, to enhance the multi-step electrocatalysis reaction.

