Balancing the Kinetic and Thermodynamic Synergetic Effect of Doped Carbon Molecular Sieves for Selective Separation of C2H4/C2H6
Abstract
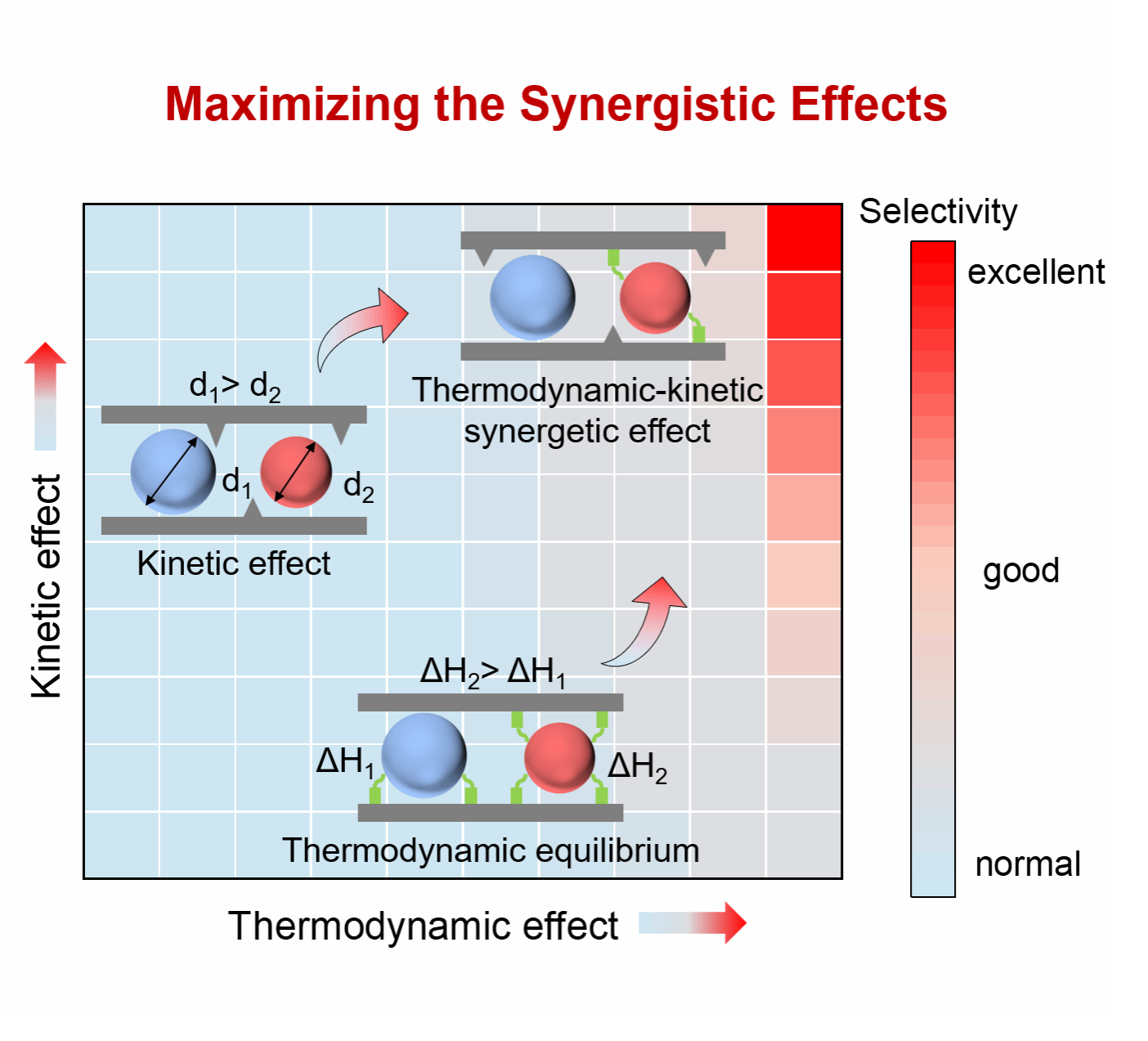
Selective separation of ethylene and ethane (C2H4/C2H6) is a formidable challenge due to their close molecular size and boiling point. Compared to industry-used cryogenic distillation, adsorption separation would offer a more energy-efficient solution when an efficient adsorbent is available. Herein, we report a class of C2H4/C2H6 separation adsorbents, doped carbon molecular sieves (d-CMSs) which were prepared from the polymerization and subsequent carbonization of resorcinol, m-phenylenediamine, and formaldehyde in ethanol solution. We demonstrated that the polymer precursor themselves could be a versatile platform for modifying the pore structure and surface functional groups of their derived d-CMSs. The high proportion of pores centered at 3.5 Å in d-CMSs contributes significantly to achieving a superior kinetic selectivity of 205 for C2H4/C2H6 separation. The generated pyrrolic-N and pyridinic-N functional sites in d-CMSs contribute to a remarkable elevation of Henry selectivity to 135 due to the enhancement of the surface polarity in d-CMSs. By balancing the synergistic effects of kinetics and thermodynamics, d-CMSs achieve efficient separation of C2H4/C2H6. Polymer-grade C2H4 of 99.71 % purity can be achieved with 75% recovery using the devised d-CMSs as reflected in a two-bed vacuum swing adsorption simulation.

