pH-dependent growth of alumina nanorods with a high aspect ratio for enhanced propane dehydrogenation performance
Abstract
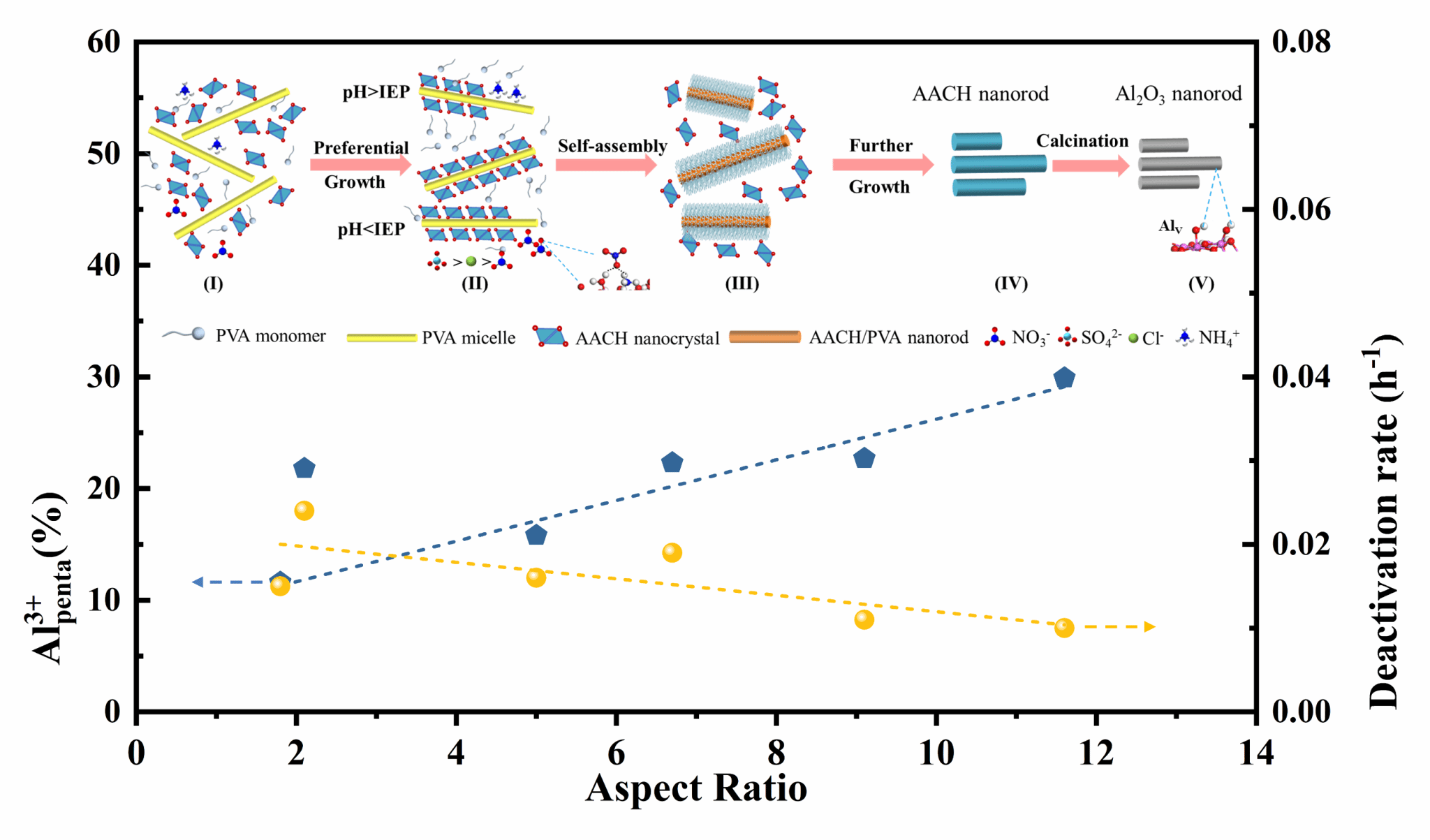
Controllable synthesis of porous alumina is always challenging due to the diversity of precursors and the complexity of the growth process. In this work, alumina nanorods with adjustable aspect ratios were synthesized by precipitation to investigate the morphology evolution mechanism related to aging pH, and the structure-activity relationship of alumina. The evolution path of precursors ammonium aluminum carbonate hydroxide (NH4Al(OH)2CO3, AACH) was established by tracking the variation in electrical charges and nitrate concentration during the growth process. It was revealed that the anisotropic adsorption of ions on the AACH crystal facets can modulate the axial and radial growth rates of the microcrystalline rods, regulating the one-dimensional oriented assembly of AACH. Alumina synthesized at pH 9.5 exhibited more exposition of (100) facets due to its higher aspect ratio, resulting in an aluminum content of up to 30% in the five-coordinated state. Due to the presence of strong metal-support interactions, the corresponding PtSn/Al2O3 catalyst demonstrated good propane dehydrogenation activity and stability, with propylene formation with 99% selectivity within 30 hours of reaction. This study highlighted the significance of pH regulation in the growth environment of AACH, enabling the selective exposure of crystal facets in the alumina support, and providing an effective pathway to obtain highly active and stable catalysts.

