Efficient and green synthesis of 2,3-benzofuran via gas-solid catalytic route
Abstract
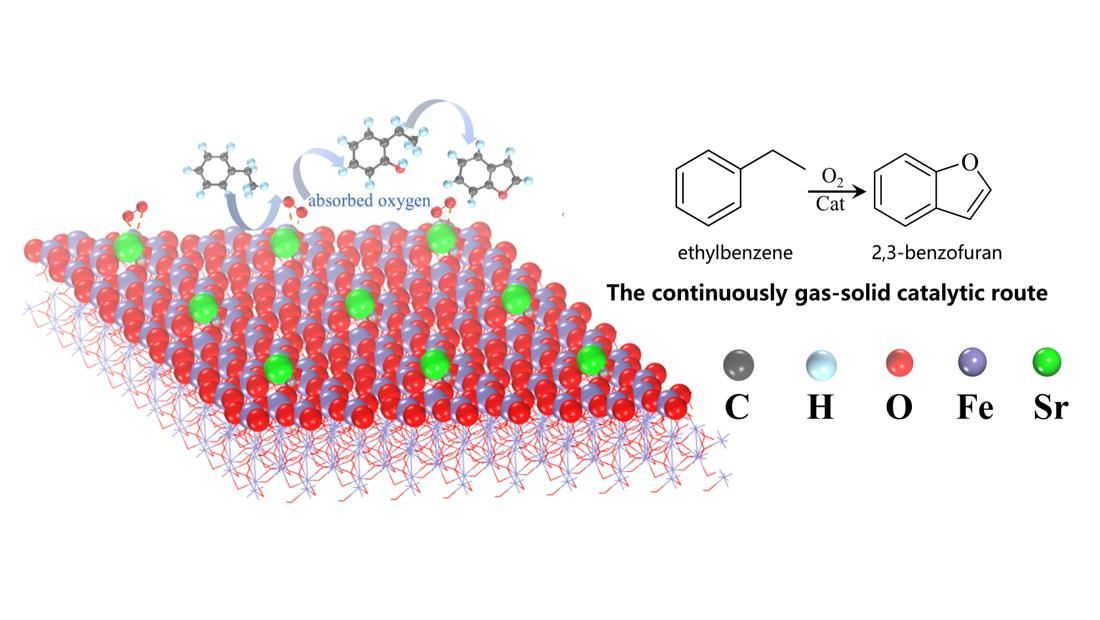
Biologically active molecule 2,3-benzofuran is an important category of heterocyclic compounds for pharmacological agents, which is usually produced via multistep organic synthesis routes under acidic, alkaline, or halogen conditions. It remains a challenge to develop a green approach for the production of 2,3-benzofuran. Herein, we report an unprecedent green synthesis of 2,3-benzofuran with co-production of styrene in the selective oxidation of ethylbenzene reaction over Sr2+ modified FeOx/SiO2 catalyst. Characterizations with O2-TPD, UV-Vis, XANES, and XRD demonstrate that the addition of Sr2+ increased the content of adsorbed oxygen and dispersion of the iron species. Kinetic investigations and DFT calculations reveal that firstly ethylbenzene reacted with oxygen adsorbed on the Fe3+ species to form the intermediate 2-ethylphenol, followed by selective oxidation of 2-ethylphenol to efficiently generate 2,3-benzofuran. These discoveries present an efficient pathway to produce 2,3-benzofuran and offer novel insights into the synthesis of furan rings, thereby contributing to advancement in chemical synthesis processes.

