Unraveling the role of Li and Mg substitution in layered sodium oxide cathodes for sodium-ion batteries
Abstract
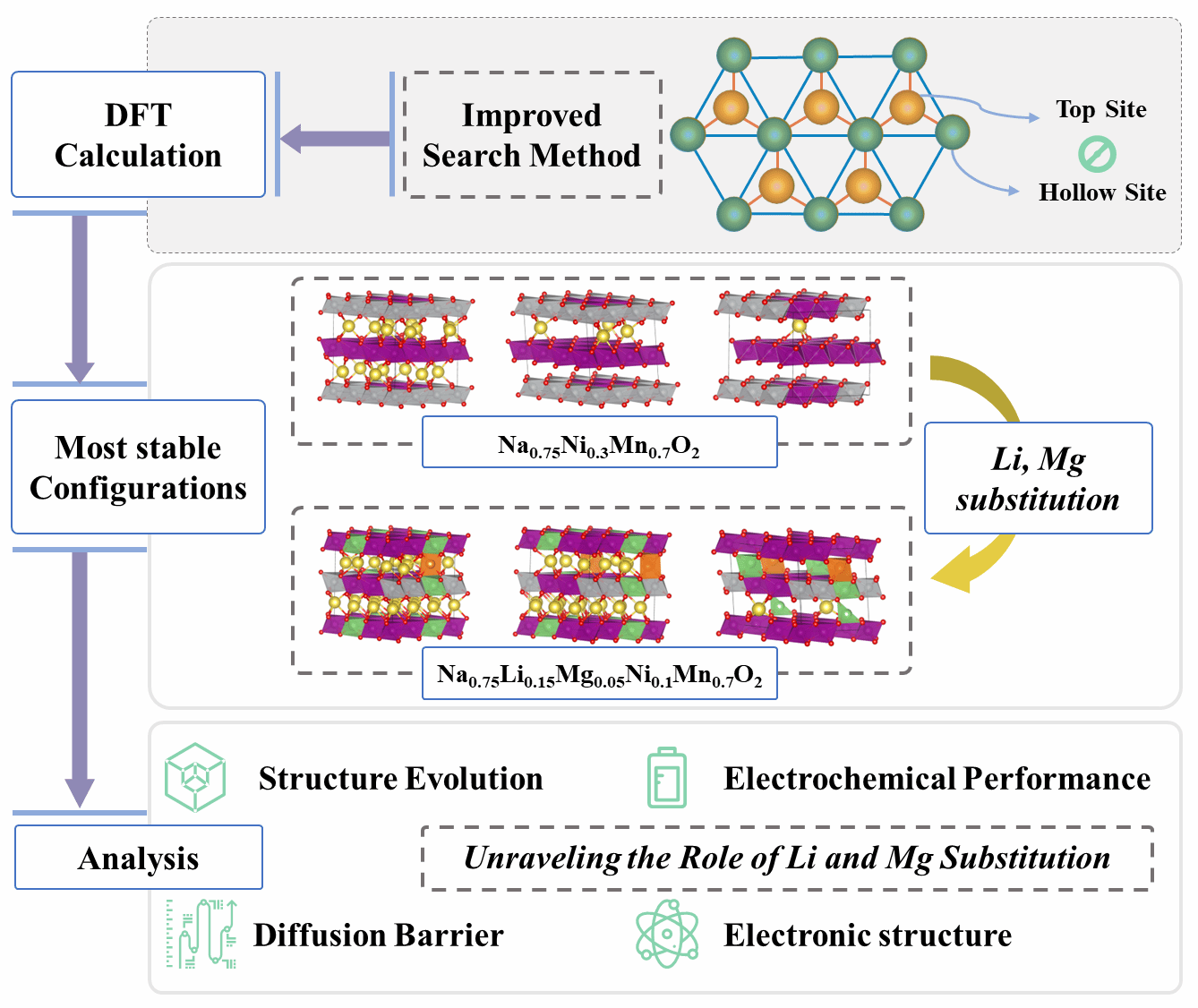
Substituting electrochemically active elements like Li and Mg in P2-type layered sodium oxide is an effective strategy for developing competitive cathode materials for sodium-ion batteries (SIBs). However, the lack of atomic-level understanding regarding the distribution of substitution positions complicates our comprehension of the roles of substituting atoms and the mechanism of sodium ion intercalation. In this study, we identified the stable configurations of Na in Na0.75Ni0.3Mn0.7O2 and Na0.75Li0.15Mg0.05Ni0.1Mn0.7O2 materials using the site exclusion method. Through simulating the complete charging process for both materials, the structure evolution of the cathodes during the cycling and the impact of the partial substitution of Ni elements by Li and Mg atoms was comprehensively elucidated. Our findings revealed that Mg atoms effectively regulate the distribution of forces within the materials, essentially serving as supportive pillars within the cathode. Meanwhile, Li atoms efficiently mitigated electron localization, consequently diminishing volume fluctuations during the charging process. More importantly, the substitution with Li and Mg atoms could synergistically reduce the interaction between transition metals and sodium ions, thereby reducing the diffusion energy barrier of Na ions. This study not only enhances comprehension of substituted metal atoms in P2 layered oxides, but also offers new insights for the development of sodium-ion cathode materials.

