Boron-containing MFI zeolite: microstructure control and its performance of propane oxidative dehydrogenation
Abstract
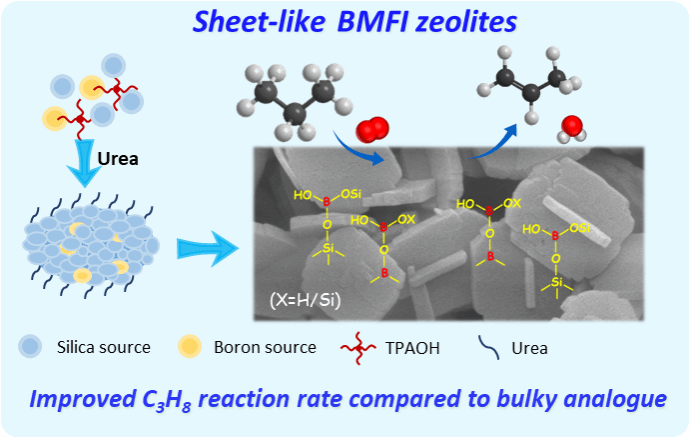
Boron-containing zeolites can catalyze the oxidative dehydrogenation of propane (ODHP) to produce propylene. Enhancing the quantity of active boron-oxygen species and regulating the positioning of these species within the zeolite are the main challenges in developing efficient boron-based catalysts. In this study, a boron-containing zeolite catalyst with exposed (010) crystal facets, referred to as the MFI-type boron-containing zeolite (BMFI), was synthesized using a urea-assisted hydrothermal method. The research indicates that the addition of an appropriate amount of urea can regulate the morphology of the zeolite, with its short-axis flake-like structure enhancing the accessibility of active boron sites and anchoring a higher content of active boron-oxygen species through hydrogen bonding, which significantly improves the ODHP activity and olefin selectivity of the catalyst. The propane conversion rate reached 20%, with a propylene selectivity of 62.3% and a total olefin selectivity of 81.3% at 520°C. Compared to the ellipsoidal boron-containing catalyst formed without urea, the flake-like BMFI catalyst exhibited nearly a 20-fold increase in the reaction rate of propane. The flake-like BMFI possesses a greater number of framework tetrahedrally coordinated boron (B[4]) and defective boron species (B[3]a and B[3]b), and active boron structural evolution occurred during the reaction process, with B[3]a and B[3]b being the active sites for the catalytic reaction. This study provides a reference for the structural design and regulation of boron-based catalysts for the oxidative dehydrogenation of light alkanes.

