Generating cationic nickel clusters over oxygen-functionalized boron nitride to boost methane dry reforming
Abstract
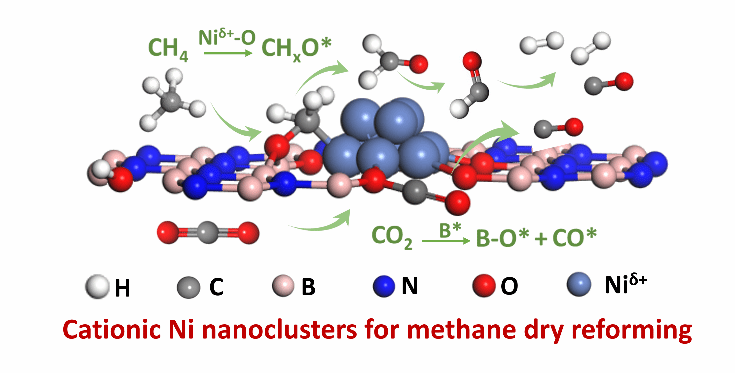
Ni-based catalysts are the most promising candidates for methane dry reforming (MDR) reaction, but still suffer from deactivation caused by coke-deposition. Herein, we reported a coke-resistance MDR catalyst consists of cationic Ni clusters (Niδ+, 0<δ<2) stabilized over oxygen-functionalized boron nitride. A simple method was developed for the fabrication of highly dispersed cationic Ni clusters on boron nitride. The local coordination modes between nickel and boron nitride determine the formation of cationic Ni clusters. We provided detailed spectroscopy and microscopy characterizations to reveal the structure features of these cationic Ni clusters. The average particle size of these clusters is ~1.7 nm. There are abundant Nixδ+-O-B interfaces on the cationic Ni clusters, which serve as catalytic active sites. Compared to conventional metallic Ni nanoparticles, the cationic Ni clusters exhibited comparable apparent catalytic activity and reduced carbon deposition, particularly operated at 600 °C where unavoidable coke formation from CH4 cracking and Boudouard reaction usually tends to occur thermodynamically and kinetically. Based on theoretical and experimental evidence, a dynamic synergistic conversion mechanism for CH4 and CO2 on Nixδ+-O-B interface has been revealed. The oxygen within Nixδ+-O-B interface could rapidly convert CHx* (x=1-3) intermediates forming H2 and CO to avoid carbon deposition. The CO2 is efficiently activated at boron sites of the Niδ+-O-B interface to regenerate active oxygen species, thereby boosting the conversion of CH4. These insights may shine light on the development of intrinsic coke-free Ni-based catalysts for methane dry reforming in the near further.

