Evolution of the active phase of Pt/Sn−Al2O3 catalysts during acidic impregnation and their use in propane dehydrogenation
Abstract
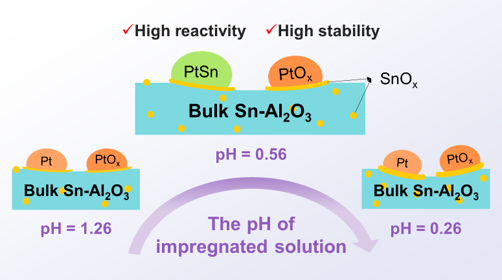
Alumina-supported PtSn is industrialized catalyst for propane dehydrogenation. During the catalyst impregnation, the acidic impregnation solution with chloroplatinic acid as a precursor would inevitably lead to the partial dissolution of the surface of amphoteric alumina support, and finally varies catalytic performance. Herein, the structure evolution of the active phase, induced by impregnated acidic solution, was studied with special care. According to the diffused double layer theory, we proposed a model of microgels during impregnation. The microgels formed in the solution with suitable acidity on the surface of the catalysts evolved into a structure of Al2O3-coated oxidized Pt by reprecipitation during drying and calcination. The covered Pt species could be exposed by Ar+ sputtering or migrate to the surface during reduction to serve as active sites for propane dehydrogenation. Noticeably, the surface Sn0 species was generated when the pH of the impregnated solution was around 0.56, which is a solid proof for the unique active phase with the PtSn alloy presented on SnOx species existing on the surface of Sn-Al2O3 support. The synthesized catalyst exhibited high propylene selectivity (99.4%) and superior stability (kd = 0.002 h-1). This study provides a new insight for the precise preparation of Pt/Sn-Al2O3 catalysts.

