Revealing an extended adsorption/insertion-filling sodium storage mechanism in petroleum coke-derived amorphous carbon
Abstract
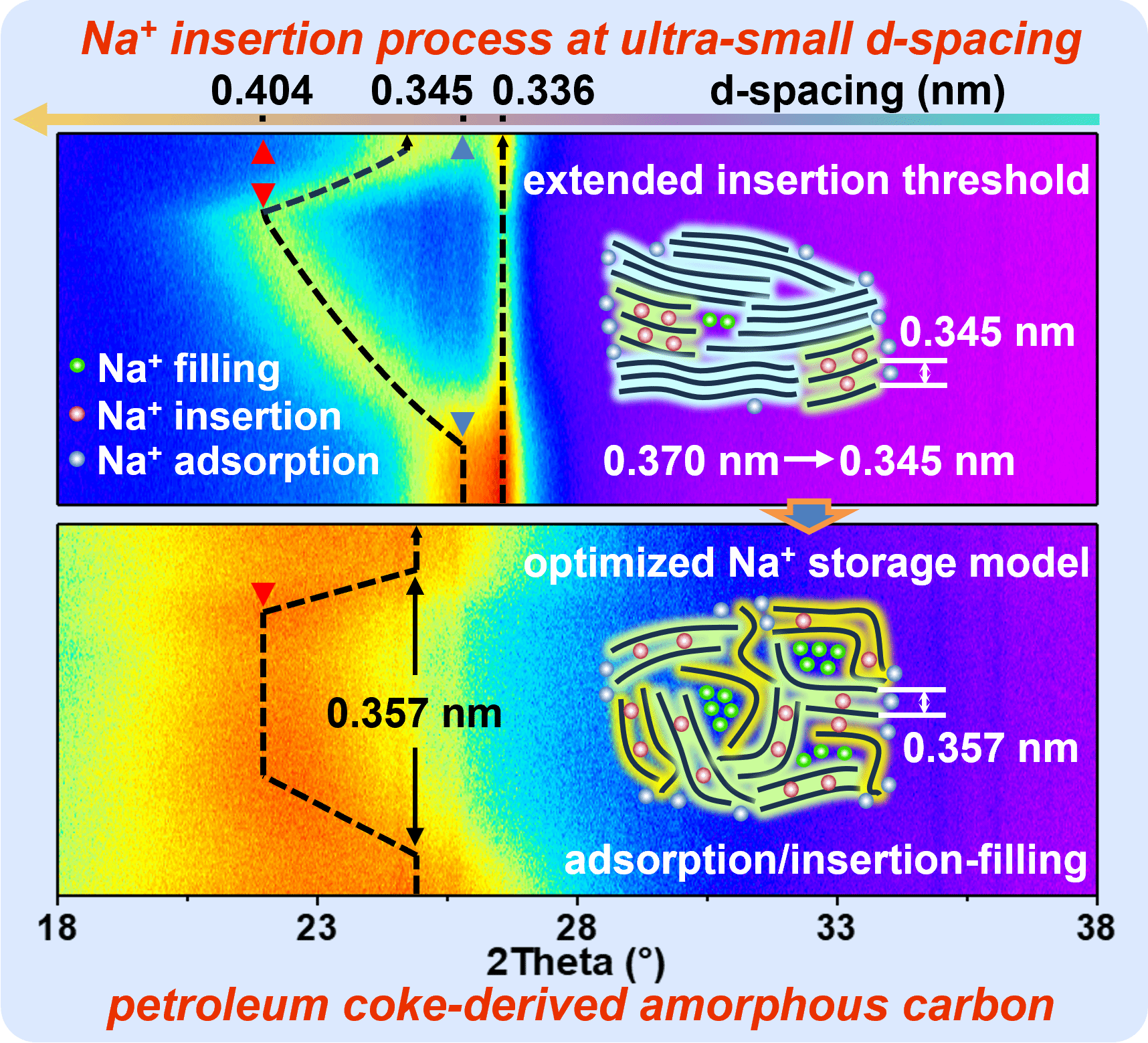
Amorphous carbon holds great promise as anode material for sodium-ion batteries due to its cost-effectiveness and good performance. However, its sodium storage mechanism, particularly the insertion process and origin of plateau capacity, remains controversial. Here, an extended adsorption/insertion-filling sodium storage mechanism is proposed using petroleum coke-derived amorphous carbon as a multi-microcrystalline model. Combining in situ X-ray diffraction, in situ Raman, theoretical calculations and neutron scattering, the effective storage form and location of sodium ions in amorphous carbon are revealed. The sodium adsorption at defect sites leads to a high-potential sloping capacity. The sodium insertion process occurs in both the pseudo-graphite phase (d002 > 0.370 nm) and graphite-like phase (0.345 ≤ d002 < 0.370 nm) rather than the graphite phase, contributing to low-potential sloping capacity. The sodium filling into accessible closed pore forms quasi-metallic sodium clusters, contributing to plateau capacity. The threshold of the effective interlayer spacing for sodium insertion is extended to 0.345 nm, breaking the consensus of insertion interlayer threshold and enhancing understanding of closed pore filling. The extended adsorption/insertion-filling mechanism explains the sodium storage behavior of amorphous carbon with different microstructures, providing theoretical guidance for the rational design of high-performance amorphous carbon anodes.

