Emergence of meta-methylbenzyl alcohol in novel pathway of ethanol with methacrolein catalyzed by hydroxyapatite
Abstract
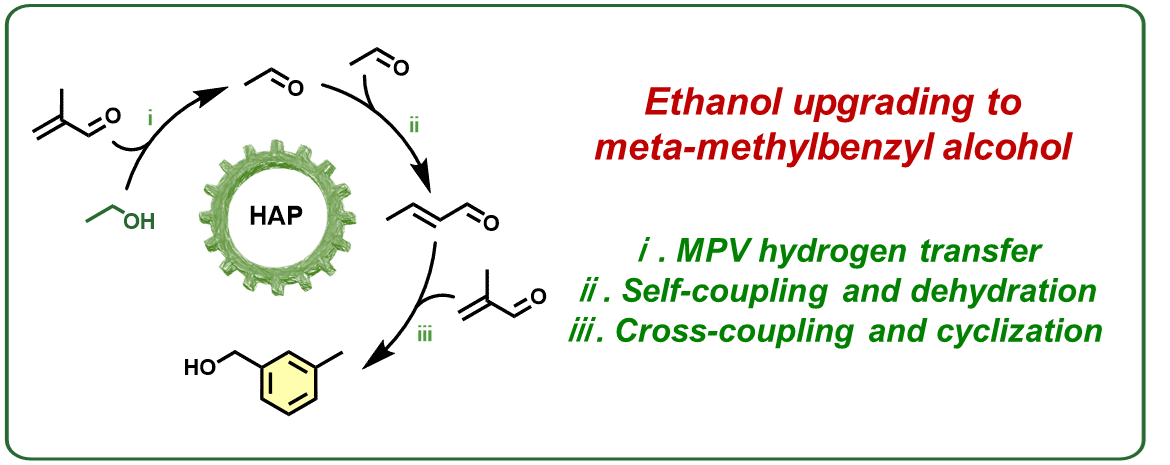
Bioethanol, a renewable resource, can be converted into high value-added chemicals. Methylbenzyl alcohol in its ortho- and para-isomers has been successfully synthesized from ethanol upgrading over metal-modified hydroxyapatite (HAP). However, meta-methylbenzyl alcohol (3-MB-OH), a particularly valuable fine chemical, has yet to be produced from ethanol. Herein, we present a novel reaction pathway for the selective synthesis of 3-MB-OH over HAP by co-feeding ethanol with methacrolein. Notably, the selectivity of 3-MB-OH reached up to 36.2% in our tests. Experiments using intermediates as reactants proved that the introduction of methacrolein definitively led to the production of 3-MB-OH, which was also confirmed by in-situ diffuse reflectance infrared Fourier-transform spectroscopy. Moreover, we inferred the co-conversion reaction pathway of ethanol and methacrolein. Density functional theory calculations indicated that ethanol preferentially underwent Meerwein-Ponndorf-Verley hydrogen transfer with methacrolein, resulting in acetaldehyde formation and subsequently promoting the synthesis of aromatic compounds.

