Revealing the origins of superior ion diffusion in biphasic layered oxide cathode for sodium-ion batteries
Abstract
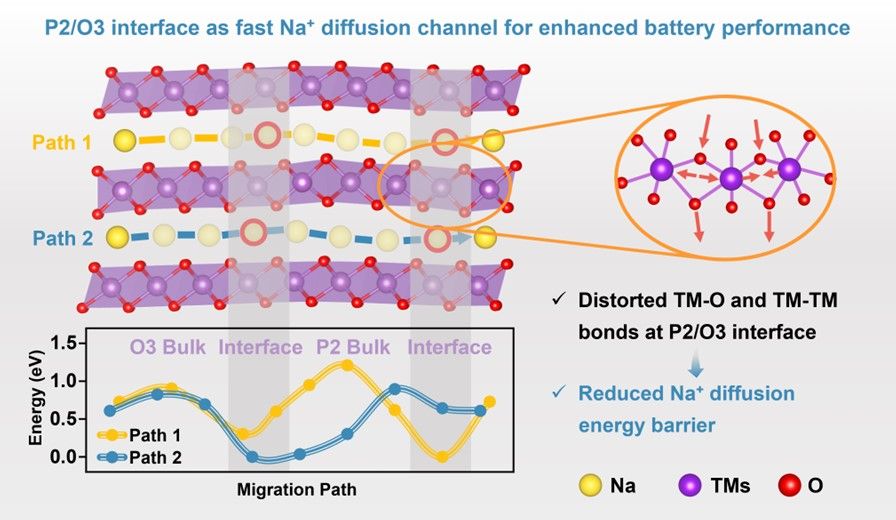
P2/O3 biphasic materials have emerged as competitive candidates for high-performance sodium-ion battery cathodes, and a thorough understanding of the ion diffusion behavior in biphasic structures particularly at phase interface is critical for unlocking their full potential. Herein, the NaxZn0.07Ni0.30Mn0.53Ti0.10O2 cathodes with finely-tuned P2/O3 phase ratios are designed and their ion diffusion mechanism is revealed through in-depth structural-electrochemical investigation. Experiments verify that a balanced phase composition of P2/O3-Na0.82 with 52.83% P2 and 47.17% O3 can maximize the coupling advantages of the biphasic structure and exhibit excellent Na+ diffusion kinetics, delivering a remarkable rate capability (143.0 mAh g-1 at 0.2C, 100.2 mAh g-¹ at 10C with 70.1% retention), outperforming most reported P2/O3 biphasic cathodes. High-resolution transmission electron microscopy and X-ray absorption fine structure results indicate that the coordination environment of Ni-O and Ni-TM paths undergoes conspicuous local symmetry breaking, driving the P2/O3-Na0.82 interface structure distortions, which exhibit unique characteristics distinct from single-phase systems. Theoretical analyses reveal that the interfacial distortions structures facilitate the overlap of Na+ energy distributions and create interconnecting bridges across different Na+ sites, ultimately promoting low-energy-barrier Na+ diffusion. These findings establish an atomic-level insight into the interface-induced ion diffusion acceleration mechanism in biphasic materials.

