High-Selective Upgrading of Ethanol to C4-10 Alcohols over Hydroxyapatite Catalyst with Superior Basicity
Abstract
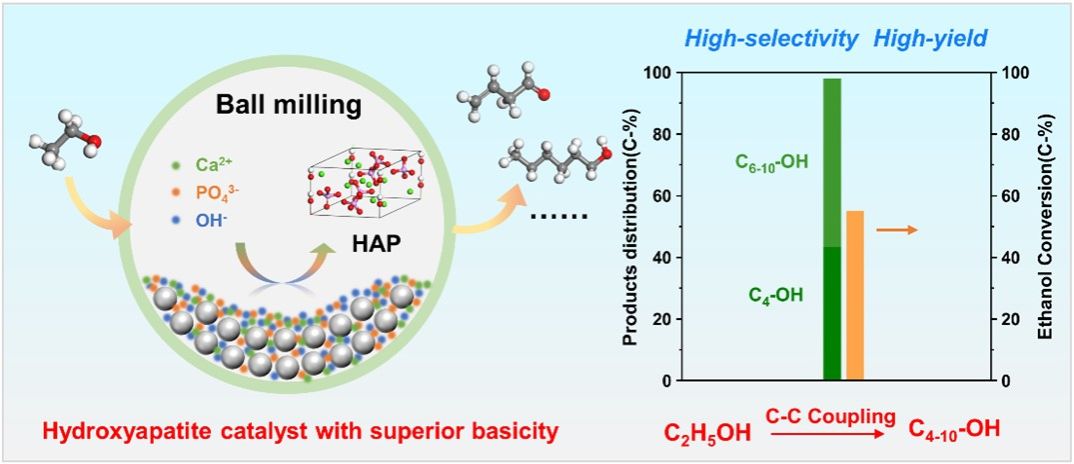
The catalytic upgrading of renewable ethanol to C4-10 alcohols via C-C coupling offers a green and negative-carbon emission pathway toward value-added compounds. The manipulation of catalysts’ surface basic and acidic properties is the key to achieve high selective of C4-10 alcohols. In this study, we present a solvent-free mechanochemical approach for the synthesis of hydroxyapatite (HAP) catalysts with enhanced basicity. The selectivity for a total C4-10 alcohols reaches 97.8% with yield of 53.9% at 325 °C and 0.1 MPa, surpassing previously reported catalysts in the literature. The mechanochemically synthesized HAP catalysts extend along the c-axis and expose the (002) crystal plane with enriched strong basic [Ca-O-P] sites. CO2-TPD and XPS analyses results demonstrated that the hydrogen bonds between the oxygen atoms of adjoining phosphate groups enhance the basic property of the catalyst surfaces. The kinetic measurements have demonstrated that the abundance of strong basic sites facilitates the adsorption of ethanol molecules and accelerates the rate of C-C coupling reactions, being responsible for a high yield of C4-10 alcohols. This work offers a sustainable approach for synthesizing such alcohols and stimulates the advancement of environmentally friendly catalysts.

