“Selective oxygen-doping in self-supportive carbon electrode by electrochemical oxidation for electrocatalytic synthesis of hydrogen peroxide”
Abstract
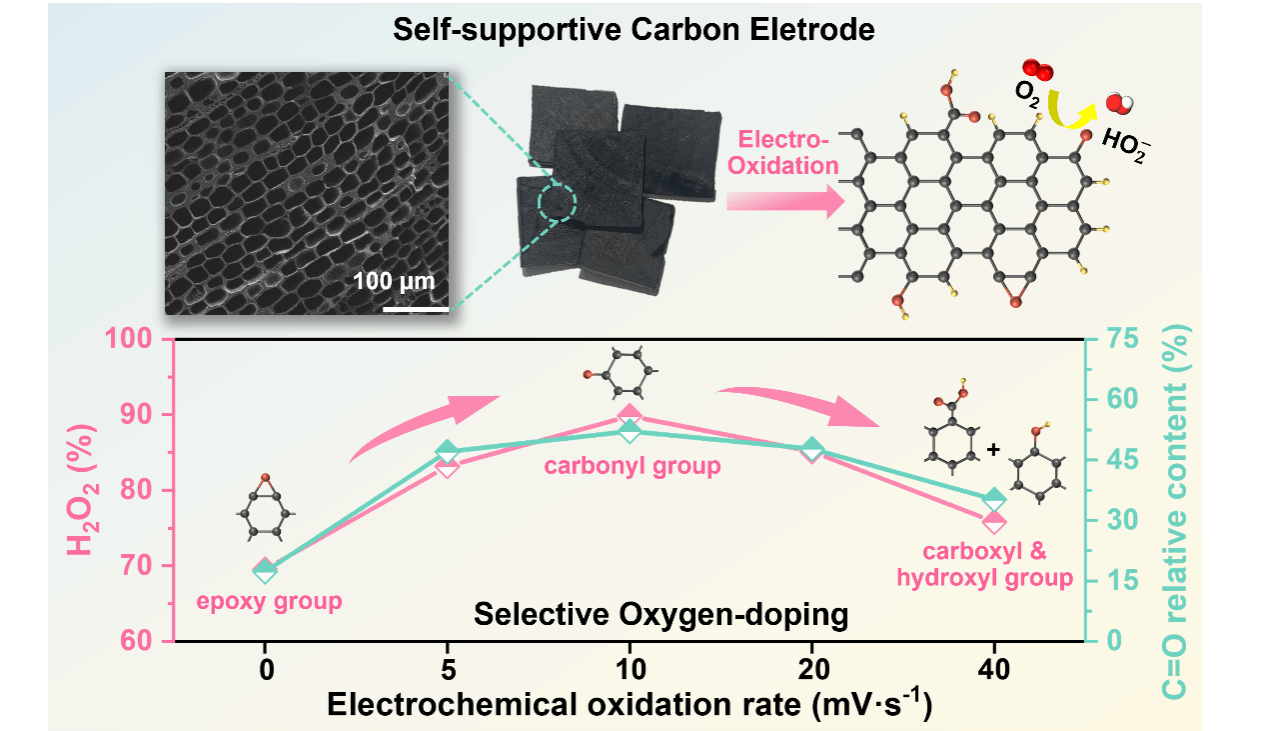
We report the selective introduction of oxygen-containing functional groups for two-electron oxygen reduction reaction (2e-ORR) in self-supportive carbon electrode using electrochemical oxidation method. The optimal electrode shows hydrogen peroxide (H2O2) selectivity up to 95%. C=O is found active to control 2e-ORR selectivity and mechanism of oxygen species variation is elucidated by in situ infrared reflection absorption spectroscopy. We further improve mass transfer by introducing highly interconnected macropores in electrode, which shows H2O2 yield of 144 mmol cm-2 h-1 and Faradaic efficiency of nearly 100%. These may inspire advanced active electrode creation for applications through selective electrochemical doping and structural design.

